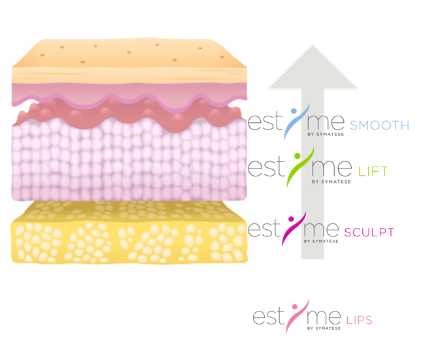
ESTYME® FILLERS products are unique integrated solutions [gel + delivery system] on the market, jointly developed by SYMATESE research. At the heart of the products, a proprietary technology created by our researchers and resulting from our expertise in hyaluronic acid: C-HA.PURE TECHNOLOGY by SYMATESE™. A technology that makes it possible to develop highly targeted products, each dedicated to an indication of the face. Each product ESTYME® SMOOTH, LIPS, LIFT & SCULPT has specific characteristics and responds exactly and effectively to the needs of the area concerned.

 |  |  |  | |||
|---|---|---|---|---|---|---|
INTENDED PURPOSE
| Modification of the skin anatomy and facial appearance. (Non-medical purpose)
| |||||
INDICATIONS | Indicated to correct nasolabial folds and perioral lines | Indicated to correct the volume and the shape of the lips
| Indicated to correct nasolabial folds
| Indicated to restore cheek volume loss
| ||
TISSUES LEVEL OF INJECTION | Dermis to hypodermis | Dermis, hypodermis and labial mucosa
| Dermis to hypodermis
| Hypodermis and supraperiosteal | ||
COLD TECHNOLOGY by SYMATESE based on the following key elements:
The gel of each ESTYME® FILLERS Product is sterile, bioabsorbable, colorless made of crosslinked hyaluronic acid, non-crosslinked hyaluronic acid from non-animal sources and containing lidocaine hydrochloride 3 mg/mL.
Each ESTYME® FILLERS Product contains local anesthetic lidocaine in order to reduce pain and improve comfort during and post-injection.
The gel is supplied in a 1 mL plastic syringe which is the sterile barrier system. The gel is sterilized by moist heat (air/steam mixture). The syringe containing the sterile gel is assembled with plunger rod and finger rest and corresponds to the injection device. The needles supplied are sterilized by radiation.
Each ESTYME® FILLERS Product is available in boxes of one or two protective blister(s) including an injection device and two ultra-thin wall needles.The injection device and the needles are for single use only.
ESTYME® dermal fillers are intended for non-medical use only.
ESTYME® dermal fillers are intended to modify the skin anatomy and facial appearance.
 |  |  |  |
|---|---|---|---|
Indicated to correct nasolabial folds and perioral lines.
Injected into dermis to hypodermis. |
Indicated to correct the volume and the shape of the lips.
Injected into dermis, hypodermis and labial mucosa.
|
Indicated to correct nasolabial folds.
Injected into dermis to hypodermis. |
Indicated to restore cheek volume loss.
Injected into hypodermis and supraperiosteal |
 |  |  |  |
Each ESTYME® FILLERS Product is contraindicated in:
Each ESTYME® FILLERS Product is used in adult subjects, excluding pregnant and breastfeeding women. Each ESTYME® FILLERS Product is not to be used in persons who are less than 18 years old.
 |  |  |  |
|---|---|---|---|
On average, with 1,2 mL injected in the nasolabial folds and 0.5 ml injected in lip fine lines, in one session, between 90 and 93% of subjects are noticeably improved at 9 months (clinical study results obtained with the Global Aesthetic Improvement Scale)3.
The expected lifetime for ESTYME ® SMOOTH is 12 months. | On average, with 1 mL injected in the lips in one session, between 90 and 93% of subjects are noticeably improved at 9 months (clinical study results obtained with the Global Aesthetic Improvement Scale)3.
The expected lifetime for ESTYTME ® LIPS is 9 months | On average, with 0.9 mL injected per nasolabial fold in one session, 82% of subjects are noticeably improved at 9 months (clinical study results obtained with the Global Aesthetic Improvement Scale)4.
The expected lifetime for ESTYME® LIFT is 9 months | On average, with 1,1 mL injected per cheek in one session, between 58.2 and 60% of subjects are noticeably improved at 9 months (clinical study results obtained with the Global Aesthetic Improvement Scale)5.
The expected lifetime for ESTYME® SCULPT is 18 months. |
1 FASY MDR 23-031 V0.
2 FASY 19-036 V0.
3 Internal data: prospective study evaluating lips enhancement and perioral lines correction with FASY hyaluronic acid dermal fillers (CLIN 1901).
4 Internal data: a prospective, single center, comparative, double blinded clinical study evaluating the efficacy of a new dermal filler on nasolabial folds correction (CLIN 1701).
5 Internal data: an interventional prospective study evaluating the safety and performance of FASY hyaluronic acid dermal filler in correction of cheeks volume loss (CLIN 1907).
 |  |  |  | |
|---|---|---|---|---|
Total Sodium Hyaluronate | 20 mg/g
|
20 mg/g
| 22 mg/g
| 23 mg/g
|
Lidocaine hydrochloride | 3 mg/g | 3 mg/g | 3 mg/g | 3 mg/g |


The following document describes the regulatory content of the ESTYME® Patient Page in order to answer to the article 18 of the Regulation 2017/745 concerning the Implant Card and the information to be supplied to the patient with an implanted device.


DESCRIPTION | REFERENCE | Box composition |
|---|---|---|
ESTYME® SMOOTH
(Indicated to correct nasolabial folds and perioral lines)
| SMOO1-300 | 1 blister / box including 1 injection device and 2 needles (2 x 30G½”) per blister. |
SMOO1-301 | 1 blister / box including 1 injection device and 2 needles (2 x 30G½”) per blister. | |
SMOO2-300 | 2 blisters / box including 1 injection device and 2 needles (2 x 30G½”) per blister. | |
SMOO2-301 | 2 blisters / box including 1 injection device and 2 needles (2 x 30G½”) per blister. |

DESCRIPTION | REFERENCE | Box composition |
|---|---|---|
ESTYME® LIPS
(Indicated to correct the volume and the shape of the lips)
| LIPS1-300 | 1 blister / box including 1 injection device and 2 needles (2 x 30G½”) per blister. |
LIPS1-301 | 1 blister / box including 1 injection device and 2 needles (2 x 30G½”) per blister. | |
LIPS2-300 | 2 blisters / box including 1 injection device and 2 needles (2 x 30G½”) per blister. | |
LIPS2-301 | 2 blisters / box including 1 injection device and 2 needles (2 x 30G½”) per blister. |

DESCRIPTION | REFERENCE | Box composition |
|---|---|---|
ESTYME® LIFT
(Indicated to correct nasolabial folds)
| LIFT1-300 | 1 blister / box including 1 injection device and 2 needles (1 x 30G½” and 1 x 27G½) per blister. |
LIFT1-301 | 1 blister / box including 1 injection device and 2 needles (1 x 30G½” and 1 x 27G½) per blister. | |
LIFT2-300 | 2 blisters / box including 1 injection device and 2 needles (1 x 30G½” and 1 x 27G½) per blister. | |
LIFT2-301 | 2 blisters / box including 1 injection device and 2 needles (1 x 30G½” and 1 x 27G½) per blister. |

DESCRIPTION | REFERENCE | Box composition |
|---|---|---|
ESTYME® SCULPT
(Indicated to restore cheek volume loss)
| SCUL1-300 | 1 blister / box including 1 injection device and 2 needles (2 x 27G½) per blister.. |
SCUL1-301 | 1 blister / box including 1 injection device and 2 needles (2 x 27G½) per blister. | |
SCUL2-300 | 2 blisters / box including 1 injection device and 2 needles (2 x 27G½) per blister. | |
SCUL2-301 | 2 blisters / box including 1 injection device and 2 needles (2 x 27G½) per blister. |
Products must be stored between +2°C and +25°C. Protect the gel from sunlight.
After opening, discard any unused material according to the institutional policies.
Copyright © Symatese Lab 2024