Collapat® II
COLLAPAT®II is a collagen medical device containing hydroxyapatite used as bone substitute in orthopaedics, spine surgery and in maxillo facial surgery and odonto-stomatology.

COLLAPAT® II is a bone void filler presented in a sponge form.
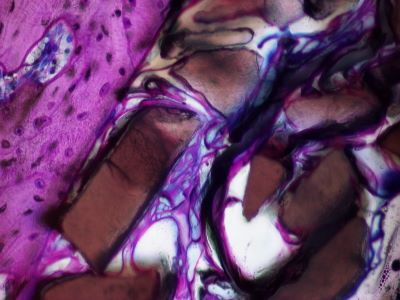
It is composed of a collagen structure in which ceramised hydroxyapatite granules are dispersed. The granules of hydroxyapatite give the material its osteoconductive properties. The hydroxyapatite is slowly resorbed.
As a collagen medical device, COLLAPAT® II has a strong haemostatic power and is completely resorbable in a few weeks.
The collagen type I is extracted from bovine dermis. The manufacturing procedure comprises stages recognized to inactivate viruses and non-conventional transmissible agents such as Prions.
Properties
- COLLAPAT® II bone void filler is osteoconductive. It is generally completely colonised by the healthy orthotopic tissue thanks to intensive bone regeneration.
- COLLAPAT® II bone void filler exerts a haemostatic effect on the bone surfaces that it covers and on the muscles that are partially freed and replaced during surgery, stopping bleeding in a few minutes.
- COLLAPAT® II bone void filler is ready to use and easy to handle, to mold and to trim.
- COLLAPAT® II bone void filler is hydrophilic: the matrix takes the consistency of a paste in contact with blood or tissue fluids.
- COLLAPAT® II bone void filler perfectly fits to the anatomic area.
Indications
In orthopaedics, COLLAPAT® II as a bone substitute is used to fill various types of bone lesions:
- After extracting cortico-spongoid bone fragments
- After tumour resection
- In revision prosthesis
- In surgical spondylodeses
- In cases of pseudarthrosis
- In certain fractures treated by osteosynthesis
Examples of usage
Specific use cases are referred in our clinical da
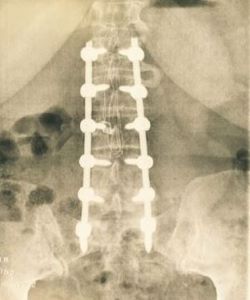
Spinal surgery
• In cervical surgery: alone inside cervical fusion cage.
• In lumbar surgery: mixed with autograft and in contact with osteosynthesis material.
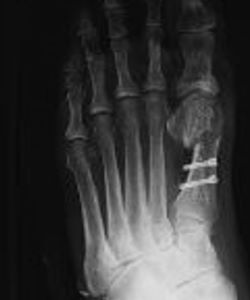
Foot surgery
in the treatment of Hallux valgus: to fill the defect created by the osteotomy, then to fill the defect after the removal of the screws.

Knee and Hip
in total prosthesis, between the sharpened bone and the prosthesis.
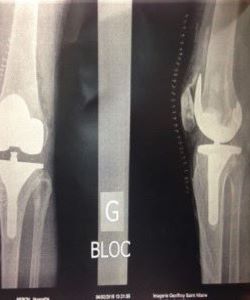
Knee in ligamentoplasty
to fill the bone graft harvesting sites and the entry to the tibial tunnel.
Method of Administration
COLLAPAT® II bone void filler must be used in perfectly sterile operating conditions after adequate preparation of the site to be treated.
COLLAPAT® II bone void filler can be cut, using surgical scissors, to the desired dimensions to facilitate its application. After being wet with tissue fluids, antibiotics or saline solution, COLLAPAT® II bone void filler becomes soft and paste-like, making it easy to use to fill the cavity requiring treatment.
Draining is strongly recommended but the drains must not be in direct contact with COLLAPAT® II.
Rinsing of the implanted area is to be avoided.
COLLAPAT® II bone void filler is not designed to be removed except in the case of post-surgical infection.
In the case of widespread and very deep bone lesions or segment defects of more than 1 to 2 cm, autologous bone shavings or PRP (Platelet Rich Plasma) should be combined with COLLAPAT® II bone void filler.
Bone instabilities require supporting osteosynthesis.

Presentation and Size
COLLAPAT® II bone void filler is available in three sizes:
REF. | DESCRIPTION | SIZE |
|---|---|---|
PAT35X6 | 3.5 x 6 x 0.6 cm | 1 unit per box |
PAT7X11 | 7 x 11 x 0.6 cm | 1 unit per box |
PAT1X1X1 | 1 x 1 x 1 cm | 5 unit per box |
COLLAPAT® II bone void filler is presented in light- and water-proof double wrapping, one unit per box, except for the 1 x 1 x 1 cm pads which are presented in boxes containing five units.
For further information please contact us.
COLLAPAT®II bone void filler is CE marked by the notified body LNE / G-MED n°0459 and is a Class III Medical Device made by SYMATESE – Chaponost – FRANCE.
Contra-indications: For full list of recommendations for use and contra-indications, method of administration, please refer to the Instruction For Use of the product included in the box.
Informations for patients

The following document describes the regulatory content of the COLLAPAT® Patient Pages in order to answer to the article 18 of the Regulation 2017/745 concerning the Implant Card.


