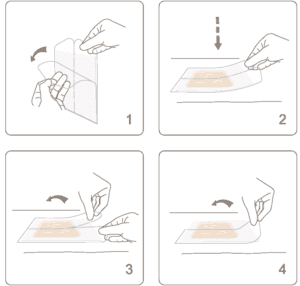
Nevelia
NEVELIA® dermal regeneration template is used to reconstruct the dermis following a skin loss.

NEVELIA® dermal regeneration template is a sterile medical device consisting of a collagen layer to promote dermal regeneration and a reinforced silicone layer acting as a pseudo-epidermis.
The key features of NEVELIA® have been developed to achieve dermal reconstruction close to native skin thanks to:
- Our expertise in the field of SKIN AND DERMAL REGENERATION
- Our SCIENTIFIC APPROACH to development and manufacturing
- Our know-how in COLLAGEN TRANSFORMATION
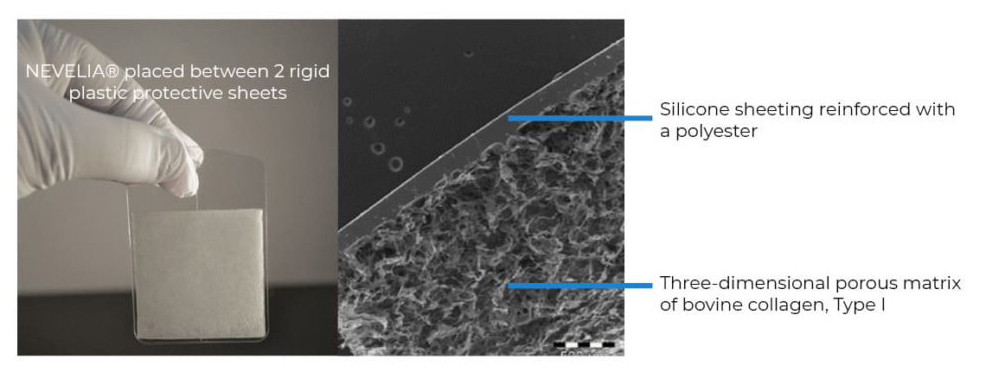
Properties
The bovine collagen type I, purified, stabilized, is supplied as a porous matrix. This dermal regeneration template serves as a support for cell infiltration, thus contributes to the natural tissue regeneration process. It is resorbed, becoming a vascularized tissue that is histologically very close to the normal dermis, from 2 to 3 weeks after it is implanted. The silicone layer is removed after dermal regeneration, at the time of the thin split thickness skin graft.
NEVELIA® is a three-dimensional porous matrix of stabilized bovine Collagen type I. NEVELIA® is made of a specific native collagen type I with a large fibrous proportion to keep cell adhesion signals and mechanical structure to support regeneration. In vitro tests demonstrate an optimized colonization as fibroblasts recognize collagen fibers.
NEVELIA® Additional features are:
- Optimized pore size (average pore size 100 μm) and open-cell structure promoting nutrients flow and fibroblasts migration
- Crosslinking rate for a balanced absorption / regeneration process
- No GAG added to keep cells attachment potential
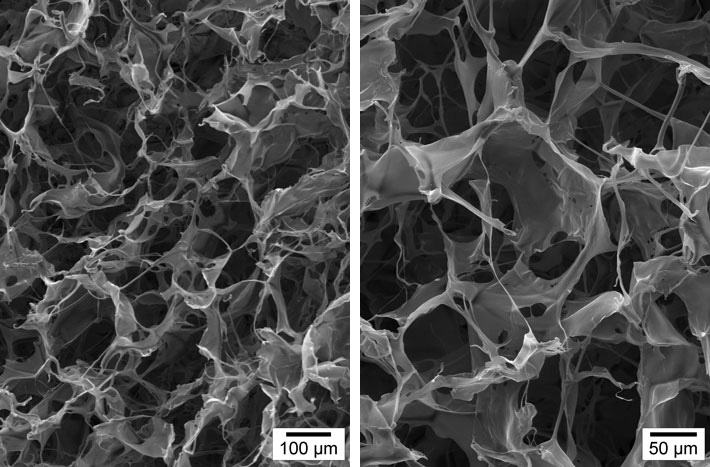
NEVELIA® MATRIX-SEM photography
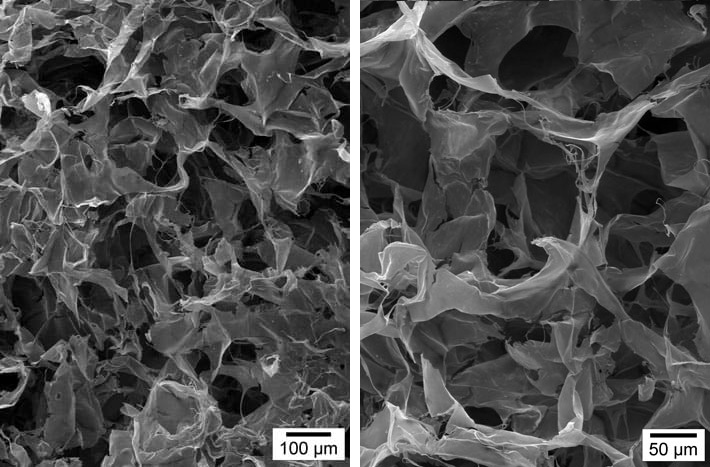
OTHER BI-LAYER MATRIX-SEM photography
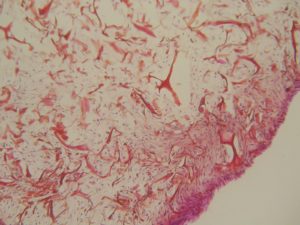
NEVELIA®
Colonisation in the matrix thickness, many cells, strong collagen neosynthesis
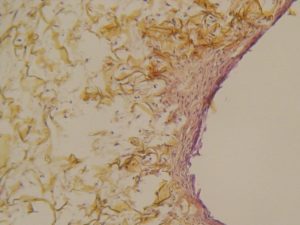
OTHER BI-LAYER MATRIX
Colonization mainly on the surface, few cells, poor collagen neosynthesis
NEVELIA® dermal regeneration template is composed of medical-grade silicone elastomer reinforced with a polyester material.

NEVELIA®
Reinforced silicone sheeting

OTHER BI-LAYER MATRIX
Non-reinforced silicone sheeting
Indications
NEVELIA® Bi-Layer Matrix is indicated for dermal regeneration in individuals with skin loss, particularly in the following fields:
- Burns surgery (third and deep second degree burns)
- Reconstructive plastic surgery
- Traumatology
NEVELIA® dermal regeneration template is used in combination with a thin split thickness skin graft (STSG) to recreate skin resembling normal skin in terms of function and appearance.
Use
- Burns
- Chronic wounds
- Traumatology
- Skyn tumors
- Others
NEVELIA® can also be used in children.
NEVELIA®, AN ALTERNATIVE TO :
- Skin Expansion (Single or sequential)
- Flaps (Local, distant, free …)
- Split-Thickness or Full-Thickness skin autograft
- Dermal graft (Allografts, Xenografts…)
Clinical benefits for specific indications

BURNS SURGERY
Waiting donor site availability for extended burns

CHRONIC WOUNDS SURGERY
Good trophicity especially necessary in areas of friction, pressure. Adapted to prolonged healing time

TRAUMATOLOGY
Good trophicity especially necessary in areas of friction, pressure. Adapted to prolonged healing time to combine other treatments.
SKIN TUMORS SURGERY
Wise choice awaiting anatomo-pathologist results. Facilitates monitoring of cancer recurrence. Ease of care for the elderly through faster mobilization.
Additional Benefits
NEVELIA® bi-layer matrix can improve treatment in a number of situations.
- Immediate availability
- Efficiency of the surgical technique
- Creation of a thicker protective tissue and reestablishment of the gliding plan in noble elements coverage like muscles, bones, tendons
- Better functional and aesthetic results compared to common dermo-epidermal graft
- No additional scars in traumatology (versus flaps)
- Reduction risk sequelae of donor site
- Does not prevent alternative treatments
- Reduction of hypertrophic scars and keloids occurrence
- Faster procedure which can be performed in ambulatory room for specific indications (hand, skin tumors)
- Children: allows better adaptation of tissue to child growth (less tensions & flanges)
Presentation and Size
This dermal regeneration template is presented in 4 sizes:
REF. | DESCRIPTION | SIZE | UNITS |
|---|---|---|---|
MCS0505 | NEVELIA® Bi-layer Matrix
for dermal regeneration | 5×5 cm | 1 |
MCS1015 | NEVELIA® Bi-layer Matrix
for dermal regeneration | 10×15 cm | 1 |
MCS1030 | NEVELIA® Bi-layer Matrix
for dermal regeneration | 10×30 cm | 1 |
MCS2030 | NEVELIA® Bi-layer Matrix
for dermal regeneration | 20×30 cm | 1 |
NEVELIA® dermal regeneration template is supplied -hydrated between two protective sheets of plastic in double pouches. Each pack contains one bi-layer matrix and is radiation-sterilized.
NEVELIA® dermal regeneration template is CE marked by the notified body G-MED n°0459 and is a Class III Medical Device made by SYMATESE – Chaponost – FRANCE
For full list of recommendations for use and contra-indications, method of administration, especially in Reconstructive plastic surgery please refer to the Instruction For Use of the product included in the box.